Aaron C. Ericsson
European Respiratory Journal 2020 55: 2000551; DOI: 10.1183/13993003.00551-2020
The manuscript by Gallacher and co-workers reveals specific bacteria associated with innate immune responses in ventilated preterm infants affected with bronchopulmonary dysplasia https://bit.ly/2Xfaq4b
First described in 1967, one of the most vexing problems in the care of preterm infants continues to be bronchopulmonary dysplasia (BPD). The clinical presentation and pathological changes associated with BPD, also referred to as chronic lung disease of prematurity, have changed substantially since that initial description by Northway et al. The condition described in that seminal report, characterised by marked respiratory distress associated with pulmonary oedema due to shunting across the patent ductus arteriosus, was also specific to preterm infants that had received high inspired oxygen concentrations for at least a week. This newly recognised form of respiratory failure was attributed to aggressive mechanical ventilation and hyper-oxygenation, as it was typified by pulmonary hypertension and oedema, and cor pulmonale. Thus, those astute observations of a clinical syndrome likely occurring in hospitals across the world led to changes in the use of mechanical ventilation in preterm infants, and innovative research resulting in ground-breaking treatments such as antenatal glucocorticoids and synthetic surfactant therapy. While these approaches in the care of preterm infants dramatically improved survival rates of preterm infants and markedly reduced the incidence of the BPD reported by Northway et al., BPD did not disappear – it just changed.
Clinical BPD in the post-surfactant era now primarily affects severely preterm infants, and results in very different lesions from classic BPD, suggesting arrested lung development rather than injury and repair. Moreover, while mortality associated with BPD is now very low, overall incidence has remained relatively steady for the past several decades, and survivors who experienced only mild respiratory distress in the perinatal period are still predisposed to late respiratory disease during childhood and adulthood, including increased risks for reactive airway disease, exercise intolerance, and other adverse sequelae. Thus, there is a continued interest in the identity and influence of genetic and environmental factors, such as adrenal insufficiency , and ante- and post-natal infections on the development of BPD.
Of particular note, three independent reports emerged in 1988 suggesting a causal relationship between Ureaplasma urealyticum in the lower airways and BPD in separate cohorts of preterm infants. While isolates from the phylum Firmicutes such as Staphylococcus and Streptococcus spp. were also reported, U. urealyticum (phylum Tenericutes) was the most common isolate from preterm infants in all three studies and was significantly associated with the development of BPD. Moreover, many of the affected infants in these reports were delivered with intact membranes suggesting in utero exposure. Ureaplasma seemed like the perfect culprit as an infectious agent driving the incidence of BPD, as it is rather difficult to culture ex vivo, stains poorly with Gram stain due to its lack of a cell wall, and is thus not susceptible to certain antibiotics commonly used to treat neonatal infections, i.e. beta-lactams. Follow-up studies documented maternal transmission to preterm infants, and pneumonic responses associated with infection.
Since those initial reports, however, a controversy of sorts developed regarding the influence of U. urealyticum in the development of BPD in preterm infants. As the first culture-independent methods of detecting specific bacteria (e.g. PCR) or profiling mixed bacterial communities (e.g. T-RFLP, DGGE) were applied to the question, associations between Ureaplasma sp. and BPD were both supported and refuted. Subsequent studies published in the past decade and employing more refined methods based on bacterial 16S rRNA gene sequences detected in endotracheal aspirates and other samples have, in general, supported an association between Ureaplasma sp. and BPD, although this finding is still not consistent across all studies. Similarly, a detailed meta-analysis of the predicted effect of azithromycin and other macrolides targeting Ureaplasma sp. on the development of BPD produced mixed results, with no clear-cut protective effect from antibiotics known to act against the clinical isolates.
Collectively, these studies simply reinforce that BPD is a multi-factorial condition. While U. urealyticum likely contributes to harmful inflammatory responses in some situations, it is not the sole aetiological agent driving the development of BPD. Additionally, these studies have occurred as part of a larger corpus of work revealing rich bacterial communities in the lungs of healthy adults. While the lower airways and lungs were not included in phase I of the Human Microbiome Project study design, based on the belief that these tissues were more or less sterile during health, it is now largely accepted that there are low biomass microbial communities constitutively present in the lungs of healthy individuals. Considering their proximity to the richly colonised oropharynx and the constant introduction of air (itself containing microbes) through the oral cavity and nares, it makes perfect sense that bacteria would reach the distal airways. It is also a testament to the mammalian immune system that the bacteria and fungi reaching our lower airways are maintained at such a low level as to render them undetectable for so many years. Indeed, earlier culture-based studies typically failed to grow the fastidious organisms detected in the lungs of healthy individuals, and there is very little evidence histologically of bacterial cells in the lungs. Highly sensitive, culture-independent methods have, however, demonstrated the presence of these bacterial communities in bronchoalveolar lavage fluid collected aseptically from healthy humans and multiple other host species. Notably, these lung communities appear to be better predictors of baseline innate immunity than oral or gut microbiotas in rodent models, and are often substantially changed in the setting of chronic respiratory disease.
In term infants, the lung microbiota is presumably established at birth via aspiration of bacteria in the oral cavity introduced from myriad environmental sources, including maternal milk microbiota , and repeatedly inoculated throughout life by subclinical micro-aspirations. Very little is known, however, regarding the ontogeny of the lung microbiota in preterm infants and the effects on inflammatory responses in the newborn. This is of great clinical significance, as maternal and fetal immune responses may have different prognostic implications for the preterm infant. Considering the reported influence of the lung microbiota on basal innate immunity in mice, it is important to consider candidate infectious agents in the context of the surrounding microbiota.
In this issue of European Respiratory Journal, Gallacher et al. describe the results of a longitudinal microbial survey of the lungs, upper airways, and stool of 55 ventilated, preterm infants from two recruitment sites, with a combined BPD incidence of 84%. Importantly, infants were assessed longitudinally to identify patterns over time. Applying qPCR and 16S rRNA-based approaches to a starting total of 1102 samples, the authors demonstrate the gradual colonisation of the upper and lower airways, punctuated by transient spikes in biomass at different sites within the first 1 to 2 weeks of life before to receding to lower baseline levels.
One of the most striking findings with regard to the detected bacterial communities was the marked dominance in the majority of samples by a single taxon. While the upper airways and lungs of full-term infants can be similarly dominated by certain taxa, they are much more likely to be colonised by relatively similar levels of multiple genera. The data presented by Gallacher et al. can perhaps be interpreted as evidence of a more permissive microbial environment in the preterm lung, wherein the first colonisers proliferate and gain a dominant position, as opposed to the term lung wherein host immune defences prevent over-proliferation of any given taxa and maintain a more balanced microbial distribution.
The authors also noted that those samples that failed to amplify and sequence adequately (a common occurrence with low biomass samples) were frequently those samples collected during prophylactic treatment with benzylpenicillin and gentamicin. Importantly, samples from children receiving antibiotics that did sequence well demonstrated no decrease in α-diversity, suggesting that treatment was broadly suppressing bacterial growth, rather than selectively killing certain taxa. With that in mind, the acute phase protein IL-6 and neutrophil chemo-attractant IL-8 were measured in each sample, and comparisons were made between samples that did or did not sequence well. While those cytokines were below the limit of detection in most fecal samples and nasopharyngeal aspirates, they were readily detectable in the tracheal aspirates and bronchoalveolar lavage fluid of preterm infants, and were significantly greater in infants providing samples that sequenced well, suggesting a link between greater levels of microbial colonisation and inflammatory responses. Focusing on that relationship over time in individual infants, there was a clear temporal relationship between microbial growth and airway inflammation, with antibiotic administration apparently mitigating both. Interestingly, the authors note that the strongest inflammatory responses were associated with specific taxa including Acinetobacter, unclassified genera within the family Enterobacteriaceae, and Mollicutes such as Mycoplasma and Ureaplasma. With that in mind, it should be noted that those latter taxa represented the dominant coloniser in a relatively small number of samples, again reinforcing the multifactorial nature of disease development.
Collectively, these data indicate that the preterm lung microbiota differs from upper airway communities, is particularly susceptible to colonisation and overgrowth by a single genus, and is significantly influenced by the dominant genus colonising the airways. Perhaps colonisation by any bacteria beyond a certain threshold invokes an inflammatory response, making BPD purely a disease of opportunity, and highlighting the limitations of Koch's postulates in the era of molecular medicine. Alternatively, perhaps select bacteria can actually provide a protective effect against opportunist agents, akin to colonisation resistance in the gut. As with those initial associations between U. urealyticum and BPD, the answers to good research always bring additional questions.

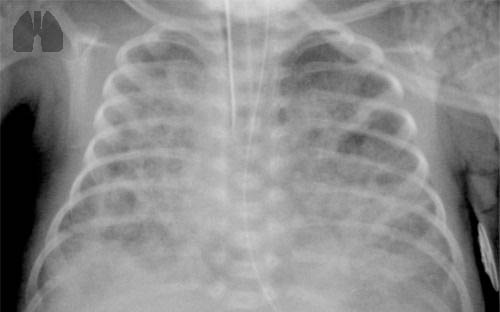
FIGURE 1
Infants with bronchopulmonary dysplasia (BPD) are typically born pre-term, and often require mechanical ventilation.
Footnotes
- Conflict of interest: A.C. Ericsson has nothing to disclose.
- Received March 4, 2020.
- Accepted April 1, 2020.
- Copyright ©ERS 2020














